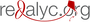Tipificación molecular de Leishmania (Leishmania) amazonensis y especies del subgénero Viannia asociadas a la leishmaniasis cutánea y la mucosa en Colombia: un estudio de concordancia
Resumen
Introducción. La electroforesis de enzimas multilocus (Multilocus Enzyme Electrophoresis, MLEE) es el estándar de referencia para la tipificación de las especies de Leishmania. La prueba está restringida a laboratorios especializados por su complejidad técnica, sus costos y el tiempo necesario para obtener resultados. La PCR-RFLP (Polymerase Chain Reaction-Restriction Fragment Length Polymorphism) se utiliza para tipificar especies de Leishmania.
Objetivo. Establecer la concordancia entre las dos pruebas como métodos de tipificación de las especies circulantes en Colombia.
Materiales y métodos. Se seleccionaron 96 aislamientos de pacientes con leishmaniasis cutánea o mucocutánea y se tipificaron mediante MLEE y PCR-RFLP con los blancos moleculares miniexon y hsp70 usados en serie. Las enzimas de restricción aplicadas fueron la HaeIII y la BccI, respectivamente. Se calculó el coeficiente kappa y un intervalo de confianza (IC) de 95 %.
Resultados. Se determinó que la concordancia fue “muy buena” al obtener un coeficiente de 0,98 (IC95%: 0,98-1,00). Las especies identificadas fueron: Leishmania Viannia braziliensis, L. (V.) panamensis, L. (V.) guyanensis y L. (L,) amazonensis. De los 96 aislamientos, 80 se enviaron a secuenciación y se confirmaron los resultados obtenidos mediante PCR-RFLP.
Conclusión. Dada la concordancia obtenida con la PCR-RFLP amplificando los genes miniexon y hsp70, se propone esta prueba como alternativa para la tipificación de especies de Leishmania circulantes en Colombia.
Descargas
Referencias bibliográficas
World Health Organization. Control of leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. Tech Rep Ser 949. Geneva: WHO; 2010. p. 1-186.
Montalvo AM, Fraga J, Aylema J, Monzote L, Montano I, Dujardin JC. PCR-RFLP/hsp70 para identificar y tipificar Leishmania de la región neotropical. Rev Cubana Med Trop. 2006;58:226-34.
del Mar-Castro M, Cossio A, Velasco C, Osorio L. Risk factors for therapeutic failure to meglumine antimoniate and miltefosine in adults and children with cutaneous leishmaniasis in Colombia: A cohort study. PLoS Negl Trop Dis. 2017;11:1-14. https://doi.org/10.1371/journal.pntd.0005515
Berzunza-Cruz M, Bricaire G, Suazo NS, Pérez-Montfort R, Becker I. PCR for identification of species causing American cutaneous leishmaniasis. Parasitol Res. 2009;104:691-9. https://doi.org/10.1007/s00436-008-1247-2
Marco JD, Barroso PA, Mimori T, Locatelli FM, Tomatani A, Mora MC, et al. Polymorphism-specific PCR enhances the diagnostic performance of American tegumentary leishmaniasis and allows the rapid identification of Leishmania species from Argentina. BMC infect Dis. 2012;12:1-8. https://doi.org/10.1186/1471-2334-12-191
Kreutzer RD, Christensen HA. Characterization of Leishmania spp. by isozyme electrophoresis. Am J Trop Med Hyg. 1980;29:199-208. https://doi.org/10.4269/ajtmh.1980.29.199
Cupolillo E, Grimaldi E, Momen H. Genetic diversity among Leishmania Viannia parasites. Ann Trop Med Parasitol. 1997;91:617-26. https://doi.org/10.1080/00034989760716
Bañuls AL, Hide M, Prugnolle F. Leishmania and the leishmaniases: A parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans.
Adv Parasitol. 2007;64:1-109. https://doi.org/10.1016/s0065-308x(06)64001-3
Cupolillo E, Momen H, Grimaldi G. Genetic diversity in natural populations of New World Leishmania. Mem Inst Oswaldo Cruz. 1998;93:663-8. https://doi.org/10.1590/s0074-02761998000500018
Stanley T, Wilson IG. Multilocus enzyme electrophoresis. Mol Biotechnol. 2003;24:203-20. https://doi.org/10.1385/1-59259-029-2:369
de Morais RC, da Costa CN, da Cunha S, de Albuquerque C, Mendonça LA, Pessoa-e-Silva R, et al. Real-time PCR for Leishmania species identification: Evaluation and comparison with classical techniques. Exp Parasitol. 2016;165:43-50. https://doi.org/10.1016/j.exppara.2016.03.005
Zhang WW, Miranda C, Arévalo J, Ndao M, Ward B, Cuentas AL, et al. Development of a genetic assay to distinguish between Leishmania Viannia species on the basis of isoenzyme differences. Clin Infect Dis. 2006;42:801-9. https://doi.org/10.1086/500326
Chaara D, Bañuls AL, Haouas N, Talignani L, Lami P, Mezhoud H, et al. Comparison of Leishmania killicki (syn. L. tropica) and Leishmania tropica population structure in Maghreb by microsatellite typing. PLoS Negl Trop Dis. 2017;9:1-13. https://doi.org/10.1371/journal.pntd.0004204
Buitrago R, Cupolillo E, Bastrenta B, Le Pont F, Martínez E, Barnabé C, et al. PCR-RFLP of ribosomal internal transcribed spacers highlights inter and intraspecies variation among Leishmania strains native to La Paz, Bolivia. Infect Genet Evol. 2011;11:557-63. https://doi.org/10.1016/j.meegid.2010.11.019
de Bruijn MH, Barker DC. Diagnosis of New World leishmaniasis: Specific detection of species of the Leishmania braziliensis complex by amplification of kinetoplast DNA. Acta Trop. 1992;52:45-58. https://doi.org/10.1016/0001-706x(92)90006-j
de Cássia R, Alheiros M, da Hora R, Rodríguez AL. Multiplex PCR as a tool for the diagnosis of Leishmania spp. kDNA and the gapdh housekeeping gene of mammal hosts. PloS One. 2017;12:1-12. https://doi.org/10.1371/journal.pone.0173922
Hamouchi A, Ejghal R, Hida M, Lemrani M. Intraspecific genetic variability in a population of Moroccan Leishmania infantum revealed by PCR-RFLP of kDNA minicircles. Acta Trop. 2017;169:142-9. https://doi.org/10.1016/j.actatropica.2017.02.016
Ovalle C, Porras-de Quintana L, Muvdi S, Ríos M. Polymerase chain reaction with two molecular targets in mucosal leishmaniasis diagnosis: A validation study. Mem Inst Oswaldo Cruz. 2007;102 549-54. https://doi.org/10.1590/s0074-02762007005000061
Mendoza-León A, Luis L, Martínez C. The β-tubulin gene region as a molecular marker to distinguish Leishmania parasites. Methods Mol Biol. 2002;179:61-83. https://doi.org/10.1385/1-59259-238-4:061
Cuervo P, Cupolillo E, Nehme N, Hernández V, Saravia N, Fernandes O. Leishmania (Viannia): Genetic analysis of cutaneous and mucosal strains isolated from the same patient. Exp Parasitol. 2004;108:59-66. https://doi.org/10.1016/j.exppara.2004.07.005
Harris E, Kropp G, Belli A, Rodríguez B, Agabian N. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-95.
Marfurt J, Nasereddin A, Niederwieser I, Jaffe CL, Beck HP, Felger I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J Clin Microbiol. 2003;41:3147-53. https://doi.org/10.1128/jcm.41.7.3147-3153.2003
Marfurt J, Niederwieser I, Makia ND, Beck HP, Felger I. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-24. https://doi.org/10.1016/s0732-8893(03)00040-3
Serin MS, Waki K, Chang KP, Aslan G, Direkel S, Otag F, et al. Consistence of miniexon polymerase chain reactionrestriction fragment length polymorphism and single-copy gene sequence analyses in discriminating Leishmania genotypes. Diagn Microbiol Infect Dis. 2007;57:295-9. https://doi.org/10.1016/j.diagmicrobio.2006.09.001
García AL, Kindt A, Bermúdez H, Llanos A, De Doncker S, Arévalo J, et al. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCRbased assay targeting heat shock protein 70 genes. J Clin Microbiol. 2004;42:2294-7. https://doi.org/10.1128/jcm.42.5.2294-2297.2004
Ovalle C, Porras L, Rey M, Ríos M, Camargo Y. Distribución geográfica de especies de Leishmania aisladas de pacientes consultantes al Instituto Nacional de Dermatología Federico
Lleras Acosta, E.S.E., 1995-2005. Biomédica. 2006:26:145-51. https://doi.org/10.7705/biomedica.v26i1.1508
Urbano J, Sánchez M, Ovalle C, Rosales MJ, Camargo Y, Gutiérrez R, et al. Characterization of cutaneous isolates of Leishmania in Colombia by isoenzyme typing and kDNA restriction analysis. Revista Ibero-latinoamericana de Parasitología. 2011;70:16-24.
Ovalle C, Díaz Y, Muvdi S. Polymerase chain reactionminiexon: A promising diagnostic method for mucosal leishmaniasis. Int J Dermatol. 2015;57:295-9. https://doi.org/10.1111/ijd.12910
Cruz ML, Ovalle C, Ortegón V, Pérez JE, Echeverry MC. Improving Leishmania species identification in different types of samples from cutaneous lesions. J Clin Microbiol. 2015;53:1339-41. https://doi.org/10.1128/jcm.02955-14
Serin MS, Daglioglu K, Bagirova M, Allahverdiyev A, Uzun S, Vural Z, et al. Rapid diagnosis and genotyping of Leishmania isolates from cutaneous and visceral leishmaniasis by microcapillary cultivation and polymerase chain reaction-restriction fragment length polymorphism of miniexon region. Diagn Microbiol Infect Dis. 2005;53:209-14. https://doi.org/10.1016/j.diagmicrobio.2005.05.007
Montalvo AM, Fraga J, Goodridge IM, Fidalgo LM, Marín M, van Der Auwera G, et al. Differentiation of Leishmania (Viannia) panamensis and Leishmania (V.) guyanensis using BccI for hsp70 PCR-RFLP. Trans R Soc Trop Med Hyg. 2010;104:364-7. https://doi.org/10.1016/j.trstmh.2009.12.002
Montalvo AM, Fraga J, Monzote L, Montaño I, De Doncker S, Dujardin JC, et al. Heat-shock protein 70 PCR-RFLP: A universal simple tool for Leishmania species discrimination in the New and Old World. Parasitology. 2010;137:1159-68. https://doi.org/10.1017/s0031182010000089
Walton BC, Shaw JJ, Lainson R. Observations on the in vitro cultivation of Leishmania braziliensis. J Parasitol. 1977;63:1118-9. https://doi.org/10.2307/3279862
Saravia N, Holguín AF, McMahon-Pratt D, D’Alessandro A. Mucosal leishmaniasis in Colombia: Leishmania braziliensis subspecies diversity. Am J Trop Med Hyg. 1985;34:714-20. https://doi.org/10.4269/ajtmh.1985.34.714
Saravia N, Segura I, Holguín AF, Santrich C, Valderrama L, Ocampo C. Epidemiologic, genetic, and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg. 1998;59:86-94. https://doi.org/10.4269/ajtmh.1998.59.86
Godfrey D, Kilgour V. Enzyme electrophoresis in characterizing the causative organism of Gambian trypanosomiasis. Trans R Soc Trop Med Hyg. 1976;70:219-24. https://doi.org/10.1016/0035-9203(76)90043-2
Wagner DB, Furnier GR, Saghai-Maroof MA, Williams SM, Dancik BP, Allard RW. Chloroplast DNA polymorphisms in lodgepole and jack pines and their hybrids. Proc Natl Acad Sci USA. 1987;84:2097-100. https://doi.org/10.1073/pnas.84.7.2097
García AL, Parrado R, De Doncker S, Bermúdez H, Dujardin JC. American tegumentary leishmaniasis: Direct species identification of Leishmania in non-invasive clinical samples. Trans R Soc Trop Med Hyg. 2007;101:368-71. https://doi.org/10.1016/j.trstmh.2006.06.009
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-74. https://doi.org/10.2307/2529310
Andrews R, Chilton N. Multilocus enzyme electrophoresis: A valuable technique for providing answers to problems in parasite systematics. Int J Parasitol. 1999;29:213-53.
https://doi.org/10.1016/S0020-7519(98)00168-4
Tsokana CN, Athanasiou LV, Valiakos G, Spyrou V, Manolakou K, Billinis C. Molecular diagnosis of leishmaniasis, species identification and phylogenetic analysis. In: Claborn DM, editor. Leishmaniasis - Trends in epidemiology, diagnosis and treatment. Croatia: InTech; 2014. p. 61-193. https://doi.org/10.5772/57067
van der Auwera G, Dujardin JC. Species typing in dermal leishmaniasis. Clin Microbiol Rev. 2015;28:265-94. https://doi.org/10.1128/cmr.00104-14
van der Auwera G, Ravel C, Verweij JJ, Bart A, Schonian G, Felger I. Evaluation of four single-locus markers for Leishmania species discrimination by sequencing. J Clin Microbiol. 2014;52:1098-104. https://doi.org/10.1128/jcm.02936-13
Saki J, Meamar A, Oormazdi H, Akhlaghi L, Maraghi S, Mohebali M, et al. Mini-exon genotyping of Leishmania species in khuzestan province, southwest Iran. Iran J Parasitol. 2010;5:25-34.
Montalvo AM, Fraga J, Maes I, Dujardin JC, van der Auwera G. Three new sensitive and specific heat-shock protein 70 PCRs for global Leishmania species identification. Eur J Clin Microbiol Infect Dis. 2012;31:1453-61. https://doi.org/10.1007/s10096-011-1463-z
Fraga JA, Montalvo M, van der Auwera G, Maes I, Dujardin JC, Requena JM. Evolution and species discrimination according to the Leishmania heat-shock protein 20 gene. Infect Genet Evol. 2013;18:229-37. https://doi.org/10.1016/j.meegid.2013.05.020
van der Auwera G, Maes I, De Doncker S, Ravel C, Cnops L, van Esbroeck M, et al. Heat-shock protein 70 gene sequencing for Leishmania species typing in European tropical infectious disease clinics. Euro Surveill. 2013;18:1-9. https://doi.org/10.2807/1560-7917.es2013.18.30.20543
Montalvo AM, Fraga J, El Safi S, Gramiccia M, Jaffe CL, Dujardin JC, et al. Direct Leishmania species typing in Old World clinical samples: Evaluation of 3 sensitive methods based on the heat-shock protein 70 gene. Diagn Microbiol Infect Dis. 2014;80:35-9. https://doi.org/10.1016/j.diagmicrobio.2014.05.012
Rodríguez I, Góngora R, Prager M, Pacheco R, Montero LM, Navas A, et al. Etiologic agent of an epidemic of cutaneous leishmaniasis in Tolima, Colombia. Am J Trop Med Hyg. 2008;78:276-82.
Fernández OL, Díaz Y, Ovalle C, Valderrama L, Muvdi S, Rodríguez I, et al. Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia. PLoS Negl Trop Dis. 2014;8:1-11. https://doi.org/10.1371/journal.pntd. 0002871
da Silva LA, dos Santos de Sousa C, da Graҫa GC, Porrozzi R, Cupolillo E. Sequence analysis and PCRRFLP profiling of the hsp70 gene as a valuable tool for identifying Leishmania species associated with human leishmaniasis in Brazil. Infect Genet Evol. 2010;10:77-83. https://doi.org/10.1016/j.meegid.2009.11.001
Hernández C, Álvarez C, González C, Ayala MS, León CM, Ramírez JD. Identification of Six New World Leishmania species through the implementation of a High-Resolution Melting (HRM) genotyping assay. Parasit Vectors. 2014;7:1-7. https://doi.org/10.1186/preaccept-1870264614134473
Cardoso G, Volpini AG, Sierra GA, Paes M, Hueb M, Porrozzi R, et al. Development and validation of PCRbased assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem Inst Oswaldo Cruz. 2012;107:664-74. https://doi.org/10.1590/s0074-02762012000500014
Camara LI, Paes M, Guerra JA, das Graças M, Coelho C, Lima B, et al. Characterization of Leishmania spp. causing cutaneous leishmaniasis in Manaus, Amazonas, Brazil. Parasitol Res. 2011;108:671-7. https://doi.org/10.1007/s00436-010-2139-9
Algunos artículos similares:
- Iveth J. González, Las metacaspasas y su rol en la vida y muerte de los parásitos protozoarios humanos , Biomédica: Vol. 29 Núm. 3 (2009)
- Ana Margarita Montalvo, Lianet Monzote, Jorge Fraga, Ivón Montano, Carlos Muskus, Marcel Marín, Simonne De Donck, Iván Darío Vélez, Jean Claude Dujardin, PCR-RFLP y RAPD para la tipificación de Leishmania neotropical , Biomédica: Vol. 28 Núm. 4 (2008)
- Marcel Marín, Yudy Alexandra Aguilar, José Robinson Ramírez, Omar Triana, Carlos Enrique Muskus, El análisis molecular y el inmunogénico sugieren la ausencia de las proteínas hidrofílicas de superficie en Leishmania (Viannia) panamensis , Biomédica: Vol. 28 Núm. 3 (2008)
- Luis Alberto Cortés, Jhon James Fernández, Especies de Lutzomyia en un foco urbano de leishmaniasis visceral y cutánea en El Carmen de Bolívar, Bolívar, Colombia , Biomédica: Vol. 28 Núm. 3 (2008)
- Rafael José Vivero, Maria Angélica Contreras-Gutiérrez, Eduar Elías Bejarano, Análisis de la estructura primaria y secundaria del ARN de transferencia mitocondrial para serina en siete especies de Lutzomyia , Biomédica: Vol. 27 Núm. 3 (2007)
- Elsa Nieves, Neudo Buelvas, Maritza Rondón, Néstor González, Las glándulas salivales de dos flebotominos vectores de Leishmania: Lutzomyia migonei (França) y Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) , Biomédica: Vol. 30 Núm. 3 (2010)
- Carlos Pérez, Yoanet Solías, Gerzaín Rodríguez, Leishmaniasis cutánea difusa en un paciente con sida , Biomédica: Vol. 26 Núm. 4 (2006)
- María Angélica Contreras, Rafael José Vivero, Eduar Elías Bejarano, Lina María Carrillo, Iván Darío Vélez, Nuevos registros de flebotomíneos (Diptera: Psychodidae) en el área de influencia del río Amoyá en Chaparral, Tolima , Biomédica: Vol. 32 Núm. 2 (2012)
- Luz Adriana Acosta, Karina Mondragon-Shem, Daniela Vergara, Andrés Velez-Mira, Horacio Cadena, Lina Maria Carrillo, Ampliación de la distribución de Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae) en el departamento de Caldas: potencial aumento del riesgo de leishmaniasis visceral , Biomédica: Vol. 33 Núm. 2 (2013)
- Margaret Paternina-Gómez, Yirys Díaz-Olmos, Luis Enrique Paternina, Eduar Elías Bejarano, Alta prevalencia de infección por Leishmania (Kinetoplastidae: Trypanosomatidae) en perros del norte de Colombia , Biomédica: Vol. 33 Núm. 3 (2013)
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |