Características de la estructura molecular de las proteínas E del virus del Zika y E1 del virus de la rubéola y posibles implicaciones en el neurotropismo y en las alteraciones del sistema nervioso
Resumen
Introducción. El virus del Zika (ZIKV) es un flavivirus con envoltura, transmitido a los seres humanos principalmente por el vector Aedes aegypti. La infección por ZIKV se ha asociado con un gran neurotropismo y con efectos neuropáticos, como el síndrome de Guillain-Barré en el adulto y la microcefalia fetal y posnatal, así como con un síndrome de infección congénita similar al producido por el virus de la rubéola (RV).
Objetivo. Comparar las estructuras moleculares de la proteína de envoltura E del virus del Zika (E-ZIKV) y de la E1 del virus de la rubéola (E1-RV), y plantear posibles implicaciones en el neurotropismo y en las alteraciones del sistema nervioso asociadas con el ZIKV.
Materiales y métodos. La secuencia de aminoácidos de la proteína E-ZIKV (PDB: 5iZ7) se alineó con la de la glucopreteína E1 del virus de la rubéola (PDB: 4ADG). Los elementos de la estructura secundaria se determinaron usando los programas Vector NTI Advance®, DSSP y POSA, así como herramientas de gestión de datos (AlignX®). Uno de los criterios principales de comparación y alineación fue la asignación de residuos estructuralmente equivalentes, con más de 70 % de identidad.
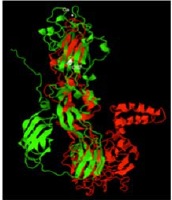
Resultados. La organización estructural de la proteína E-ZIKV (PDB: 5iZ7) fue similar a la de E1-RV (PDB: 4ADG) (70 a 80 % de identidad), y se observó una correspondencia con la estructura definida para las glucoproteínas de fusión de membrana de clase II de los virus con envoltura. E-ZIKV y E1-RV exhibieron elementos estructurales de fusión muy conservados en la región distal del dominio II, asociados con la unión a los receptores celulares de entrada del virus de la rubéola (glucoproteína de mielina del oligodendrocito, Myelin Oligodendrocyte Glycoprotein, MOG), y con los receptores celulares Axl del ZIKV y de otros flavivirus.
Conclusión. La comparación de las proteínas E-ZIKV y E1-RV es un paso necesario hacia la definición de otros factores moleculares determinantes del neurotropismo y la patogenia del ZIKV, el cual puede contribuir a generar estrategias de diagnóstico, prevención y tratamiento de las complicaciones neurológicas inducidas por el ZIKV.
Descargas
Referencias bibliográficas
Fauci AS, Morens DM. Zika Virus in the Americas-yet another arbovirus threat. N Engl J Med. 2016;374:601-4.http://dx.doi.org/10.1056/NEJMp1600297
Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951-8. http://dx.doi.org/10.1056/NEJMoa1600651
Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—Reviewing the evidence for causality. N Engl J Med. 2016;374:1981-7. http://dx.doi.org/10.1056/NEJMsr1604338
Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375:1513-23. http://dx.doi.org/10.1056/NEJMoa1605564
Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, et al. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016;19:593-8. http://dx.doi.org/10.1016/j.stem.2016.08.005
Lazear HM, Govero J, Smith AM, Platt DJ, Fernández E, Miner JJ, et al. A mouse model of zika virus pathogenesis. Cell Host Microbe. 2016;19:720-30. http://dx.doi.org/10.1016/j.chom.2016.03.010
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816-8. http://dx.doi.org/10.1126/science.aaf6116
Mécharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387:1481. http://dx.doi.org/10.1016/S0140-6736(16)00644-9
Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823-6. http://dx.doi.org/10.1126/science.aaf8505
Lucchese G, Kanduc D. Zika virus and autoimmunity: From microcephaly to Guillain-Barré syndrome, and beyond. Autoimmun Rev. 2016;15:801-8. http://dx.doi.org/10.1016/j.autrev.2016.03.020
Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus.N Engl J Med. 2016;374:1552-63. http://dx.doi.org/10.1056/NEJMra1602113
Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, et al. Structure of the thermally stable Zika virus. Nature. 2016;533:425-8. http://dx.doi.org/10.1038/nature17994
White JM, Whittaker GR. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593-14. http://dx.doi.org/10.1111/tra.12389
Kielian M. Mechanisms of virus membrane fusion proteins. Ann Rev Virol. 2014;1:171-89. http://dx.doi.org/10.1146/annurev-virology-031413-085521.
Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467-70. http://dx.doi.org/10.1126/science.aaf5316
Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, et al. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe. 2016;19:696-704. http://dx.doi.org/10.1016/j.chom.2016.04.013
Cooper LZ, Krugman S. Clinical manifestations of postnatal and congenital rubella. Arch Ophthalmol. 1967;77:434-9. http://dx.doi.org/10.1001/archopht.1967.00980020436004
Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev. 2000;13:571-87. http://dx.doi.org/10.1128/CMR.13.4.571-587.2000
Waxham MN, Wolinsky JS. A model of the structural organization of rubella virions. Rev Infect Dis. 1985;7:S133-9.
Battisti AJ, Yoder JD, Plevka P, Winkler DC, Prasad VM, Kuhn RJ, et al. Cryo-electron tomography of rubella virus. J Virol. 2012;86:11078-85. http://dx.doi.org/10.1128/JVI.01390-12
Cong H, Jiang Y, Tien P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J Virol. 2011;85:11038-47. http://dx.doi.org/10.1128/JVI.05398-11
Bairoch A, Apweiler R. The SWISS-PROT protein sequence data bank and its supplement TrEMBL. Nucleic Acids Res. 1997;25:31-6.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-80.
Sussman JL, Lin D, Jiang J, Manning NO, Prilusky J, Ritter O, et al. Protein Data Bank (PDB): Database of three-dimensional structural information of biological macromolecules. Acta Crystallogr D Biol Crystallogr.1998;54:1078-84.
Kabsch W, Sander C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577-637. http://dx.doi.org/10.1002/bip.360221211
Sander C, Schneider R. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins. 1991;9:56-68. http://dx.doi.org/10.1002/prot.340090107
Ceccaldi PE, Lucas M, Despres P. New insights on the neuropathogenicity of West Nile virus. FEMS Microbiol Lett. 2004;233:1-6. http://dx.doi.org/10.1016/j.femsle.2004.01.035
Araujo AQ, Silva MT, Araujo AP. Zika virus-associated neurological disorders: A review. Brain. 2016;139:2122-30. http://dx.doi.org/10.1093/brain/aww158
Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining bloodbrain barrier integrity. Nat Med. 2015;21:1464-72. http://dx.doi.org/10.1038/nm.3974
Vidgren G, Takkinen K, Kalkkinen N, Kääriäinen L, Pettersson RF. Nucleotide sequence of the genes coding for the membrane glycoproteins E1 and E2 of rubella virus. J Gen Virol. 1987;68:2347-57. http://dx.doi.org/10.1099/0022-1317-68-9-2347
Gros C, Linder M, Wengler G, Wengler G. Analyses of disulfides present in the rubella virus E1 glycoprotein. Virology. 1997;230:179-86. http://dx.doi.org/ 10.1006/viro.1997.8462
DuBois RM, Vaney MC, Tortorici MA, Kurdi RA, Barba-Spaeth G, Krey T et al. Functional and evolutionary insight from the crystal structure of rubella virus protein E1. Nature. 2013;493:552-6. http://dx.doi.org/10.1038/nature11741
Beasley DW, Whiteman MC, Zhang S, Huang CY, Schneider BS, Smith DR. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol. 2005;79:8339-47. http://dx.doi.org/10.1128/JVI.79.13.8339-8347.2005
Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84-6. http://dx.doi.org/10.3201/eid2101.140894
Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J ClinVirol. 2015;68:53-5. http://dx.doi.org/10.1016/j.jcv.2015.04.021
Burke RM, Pandya P, Nastouli E, Gothard P. Zika virus infection during pregnancy: What, where, and why?. Br J Gen Pract. 2016;66:122-3. http://dx.doi.org/10.3399/bjgp16X683917
Deng YQ, Zhao H, Li XF, Zhang NN, Liu ZY, Jiang T, et al. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci China Life Sci. 2016;59:428-30. http://dx.doi.org/10.1007/s11427-016-5043-4
Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21:1464-72. http://dx.doi.org/10.1038/nm.3974
Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544-57. http://dx.doi.org/10.1016/j.chom.2012.08.009
Miner JJ, Diamond MS. Understanding how Zika virus enters and infects neural target cells. Cell Stem Cell. 2016;18:559-60. http://dx.doi.org/10.1016/j.stem.2016.04.009
Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval- Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18:591-6. http://dx.doi.org/10.1016/j.stem.2016.03.012
Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19:258-65. http://dx.doi.org/10.1016/j.stem.2016.04.014
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika virus in fection in human skin cells. J Virol. 2015;89:8880-96. http://dx.doi.org/10.1128/JVI.00354-15
Chan JF, Yip CC, Tsang JO, Tee KM, Cai JP, Chik KK, et al. Differential cell line susceptibility to the emerging Zika virus: Implications for disease pathogenesis, non-vectorborne human transmission and animal reservoirs. Emer Micro Infect. 2016;5:e93.http://dx.doi.org/10.1038/emi.2016.99.
Bhattacharyya S, Zagórska A, Lew ED, Shrestha B, Rothlin, CV, Naughton J, et al. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136-47. http://dx.doi.org/10.1016/j.chom.2013.07.005
Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg. 2016;94:1362-9. http://dx.doi.org/10.4269/ajtmh.16-0111
Onorati M, Li Z, Liu F, Sousa AM, Nakagawa N, Li M, et al. Zikavirus disrupts phospho-TBK1 localization and mitosis in human neuroepithelial stem cells and radial glia. Cell Rep. 2016;16:2576-92. http://dx.doi.org/10.1016/j.celrep.2016.08.038
Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20:155-66. http://dx.doi.org/10.1016/j.chom.2016.07.002
Shao Q, Herrlinger S, Yang SL, Lai F, Moore JM, Brindley MA, et al. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143:4127-36. http://dx.doi.org/10.1242/dev.143768
Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016;387:1531-9. http://dx.doi.org/10.1016/S0140-6736(16)00562-6
Coffey JC, McDermott KW. The regional distribution of myelin oligodendrocyte glycoprotein (MOG) in the developing rat CNS: An in vivo immunohistochemical study. J Neurocytol. 1997;26:149-61.
Gold R, Hartung HP, Toyka KV. Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med Today. 2000;6:88-91.
Pettersson JH, Eldholm V, Seligman SJ, Lundkvist Å, Falconar AK, Gaunt MW, et al. How did Zika virus emerge in the Pacific Islands and Latin America? MBio. 2016;7:e01239-16. http://dx.doi.org/10.1128/mBio.01239-16.
Richard AS, Shim BS, Kwon YC, Zhang R, Otsuka Y, Schmitt K, et al. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc Natl Acad Sci USA. 2017. pii:201620558. http://dx.doi.org/10.1073/pnas.1620558114
Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587-90. http://dx.doi.org/10.1016/j.stem.2016.02.016
Algunos artículos similares:
- María Luz Gunturiz, Liliana Cortés, Ester Liliana Cuevas, Pablo Chaparro, Martha Lucía Ospina, Toxoplasmosis cerebral congénita e infección por el virus del Zika y del chikunguña: reporte de un caso , Biomédica: Vol. 38 Núm. 2 (2018)
- Wilber Gómez-Vargas, Kelly Valencia-Jiménez, Guillermo Correa-Londoño, Faiber Jaramillo-Yepes, Nuevas tabletas larvicidas de Bacillus thuringiensis var. israelensis: evaluación del efecto larvicida sobre Aedes aegypti (Diptera: Culicidae) en Colombia , Biomédica: Vol. 38 Núm. Sup. 2 (2018): Suplemento 2, Medicina tropical
- Estephania Candelo, Gabriela Caicedo, Max Feinstein, Harry Pachajoa, Microcefalia en Colombia antes de la epidemia del Zika: revisión sistemática de la literatura , Biomédica: Vol. 38 Núm. Sup. 2 (2018): Suplemento 2, Medicina tropical
- Jorge Rivera, Aura C. Rengifo, Gerardo Santamaría, Sheryll Corchuelo, Diego Álvarez-Díaz, Edgar Alberto Parra, Julián Naizaque, Jeison Monroy-Gómez, Orlando Torres-Fernández, Inmunorreacción de la infección por el virus de Zika en retina de ratones , Biomédica: Vol. 39 Núm. Supl. 2 (2019): Enfermedades transmisibles en el trópico, agosto
- Lorenzo Cáceres , Cipriano Ayarza, Damaris Bernal, Evaluación de la eficacia biológica y de la sensibilidad de Aedes aegypti a los insecticidas piretroides deltametrina y ciflutrina durante el brote del virus Zika en Kuna Yala, Panamá , Biomédica: Vol. 43 Núm. 2 (2023)
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |