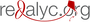El análogo de cumarina 3-metil-7H-furo[3,2-g]cromen-7-ona, un posible agente antiparkinsoniano
Resumen
Introducción. El segundo trastorno neurodegenerativo más común es la enfermedad de Parkinson. Los inhibidores de la monoamino oxidasa B se emplean en el tratamiento de esta enfermedad en monoterapia o concomitantemente con levodopa. Varios compuestos cumarínicos han mostrado actividad como inhibidores de la monoamino oxidasa B.
Objetivo. Evaluar los posibles efectos antiparkinsonianos del análogo de la cumarina FCS005 (3-methyl-7H-furo[3,2-g]chromen-7-one) en modelos de ratones, la actividad inhibitoria frente a las monoamino oxidasas (MAO) y la actividad antioxidante.
Materiales y métodos. Se sintetizó la furanocumarina FCS005 y, en los modelos de reserpina y levodopa, se evaluó si producía reversión de la hipocinesia; en el modelo de haloperidol se evaluaron sus efectos anticatalépticos. Además, se evaluó in vitro la actividad inhibidora de MAO y, ex vivo, la actividad antioxidante del compuesto FCS005.
Resultados. El compuesto FCS005 en dosis de 100 mg/kg produjo la remisión de la hipocinesia en los modelos de reserpina y de levodopa. Esta furanocumarina presentó efectos anticatalépticos con la misma dosis. Además, mostró tener actividad inhibitoria selectiva sobre la MAO B, así como efectos antioxidantes.
Conclusión. Los resultados evidenciaron propiedades interesantes del compuesto FCS005. Es importante continuar investigando esta molécula porque puede ser un potencial agente antiparkinsoniano.
Descargas
Referencias bibliográficas
Elbaz A, Carcaillon L, Kab S, Moisan F. Epidemiology of Parkinson’s disease. Rev Neurol (Paris). 2016;172:14-26. https://doi.org/10.1016/j.neurol.2015.09.012
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384-6. https://doi.org/10.1212/01.wnl.0000247740.47667.03
Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: A review. JAMA. 2014; 311:1670-83. https://doi.org/10.1001/jama.2014.3654
Alexi T, Borlongan C, Faull R, Williams C, Clark R, Gluckman P, et al. Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog Neurobiol. 2000;60:409-70. https://doi.org/10.1016/S0301-0082(99)00032-5
Emborg M. Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. J Neurosci Methods. 2004;139:121-43. https://doi.org/10.1016/j.jneumeth.2004.08.004
Gershanik OS. Improving L-dopa therapy: The development of enzyme inhibitors. Mov Disord. 2015;30:103-13. https://doi.org/10.1002/mds.26050
Finberg J. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B; focus on modulation of CNS monoamine neurotransmitter release. Pharmacol Ther. 2014;143:133-52. https://doi.org/10.1016/j.pharmthera.2014.02.010
Ariza S, Rueda D, Rincón J, Linares E, Guerrero M. Efectos farmacológicos sobre el sistema nervioso central inducidos por cumarina aislada de Hygrophila tyttha Leonard. Vitae. 2007;14:51-8.
Vergel N, López J, Orallo F, Viña D, Buitrago D, Olmo E, et al. Antidepressant-like profile and MAO-A inhibitory activity of 4-propyl-2H-benzo[h]- chromen-2-one. Life Sci. 2010;86:819-24. https://doi.org/10.1016/j.lfs.2010.04.001
Matos M, Viña D, Picciau C, Orallo F, Santana L, Uriarte E. Synthesis and evaluation of 6-methyl-3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2009;19:5053-5. https://doi.org/10.1016/j.bmcl.2009.07.039
Matos M, Viña D, Quezada E, Picciau C, Delogu G, Orallo F, et al. A new series of 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2009;19:3268-70. https://doi.org/10.1016/j.bmcl.2009.04.085
Matos M, Viña D, Janeiro P, Borges F, Santana L, Uriarte E. New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2010;20:5157-60. https://doi.org/10.1016/j.bmcl.2010.07.013
Matos M, Viña D, Vázquez-Rodríguez S, Uriarte E, Santana L. Focusing on new monoamine oxidase inhibitors: Differently substituted coumarins as an interesting scaffold. Curr Top Med Chem. 2012;12:2210-39. https://doi.org/10.2174/1568026611212200008
Matos M, Vilar S, González-Franco R, Uriarte E, Santana L, Friedman C, et al. Novel (coumarin-3-yl) carbamates as selective MAO-B inhibitors: Synthesis, in vitro and in vivo assays, theoretical evaluation of ADME properties and docking study. Eur J Med Chem. 2013;63:151-61. https://doi.org/10.1016/j.ejmech.2013.02.009
Pisani L, Farina R, Nicolotti O, Gadaleta D, Soto-Otero R, Catto M, et al. In silico design of novel 2H-chromen-2-one derivatives as potent and selective MAO-B inhibitors. Eur J Med Chem. 2015;89:98-105. https://doi.org/10.1016/j.ejmech.2014.10.029
Epifano F, Molinaro G, Genovese S, Ngomba R, Nicoletti F, Curini M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci Lett. 2008;443:57-60. https://doi.org/10.1016/j.neulet.2008.07.062
Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL, et al. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem Int. 2010;57:203-15. https://doi.org/10.1016/j.neuint.2010.05.011
Philippens I. Non-human primate models for Parkinson’s disease. Drug Discov Today Dis Models. 2008;5:105-11. https://doi.org/10.1016/j.ddmod.2008.06.004
Matos M, Rodríguez F, Borges F, Santana L, Uriarte E, Estrada M, et al. 3-Amidocoumarins as potential multifunctional agents against neurodegenerative diseases. Chem Med Chem. 2015;10:2071-9. https://doi.org/10.1002/cmdc.201500408
Aguirre P, García O, Tapia V, Muñoz Y, Cassels BK, Núñez MT. Neuroprotective effect of a new 7,8-dihydroxycoumarin-based Fe2+/Cu2+ chelator in cell and animal models of Parkinson’s disease. ACS Chem Neurosci. 2017;8:178-85. https://doi.org/10.1021/acschemneuro.6b00309
Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías SB, Del-Bel E, et al. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol. 2017;155:120-48. https://doi.org/10.1016/j.pneurobio.2015.10.004
Garazd M, Garazd Y, Ogorodniichuk A, Khilya V. Modified coumarins. Synthesis of substituted 5-(4-methoxyphenyl)-7H-furo [3,2-g] chromen-7-ones. Chem Nat Compd. 2002;38:539-48. https://doi.org/10.1023/A:1022626402415
National Center for Biotechnology Information. PubChem Open Chemistry Database Compound Summary for CID 608273. Accessed on: January 20, 2018. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/608273
Tadaiesky M, Andreatini R, Vital M. Different effects of 7-nitroindazole in reserpine-induced hypolocomotion in two strains of mice. Eur J Pharmacol. 2006;535:199-207. https://doi.org/10.1016/j.ejphar.2006.02.004
Schmidt W, Mayerhofer A, Meyer A, Kovar K. Ecstasy counteracts catalepsy in rats, an antiparkinsonian effect? Neurosci Lett. 2002;330:251-4.
Wei L, Chen L. Effects of 5-HT in globus pallidus on haloperidol-induced catalepsy in rats. Neurosci Lett. 2009;454:49-52. https://doi.org/10.1016/j.neulet.2009.02.053
Hijova E, Nistiar F, Sipulova A. Changes in ascorbic acid and malondialdehyde in rats after exposure to mercury. Bratis Lek Listy. 2005;106:248-51.
Levine R, Garland D, Oliver C, Amici A, Climent I, Lenz A, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464-78. https://doi.org/10.1016/0076-6879(90)86141-H
Baltacioglu E, Akalin FA, Alver A, Deger O, Karabulut E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch Oral Biol. 2008;53:716-22. https://doi.org/10.1016/j.archoralbio.2008.02.002
Yáñez M, Fraiz N, Cano E, Orallo F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biophys Res Commun. 2006;344:688-95. https://doi.org/10.1016/j.bbrc.2006.03.190
Colpaert F. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rats. Neuropharmacology. 1987;26:1431-40.
Kaur S, Starr M. Antiparkinsonian action of dextramethorphan in the reserpine-treated mouse. Eur J Pharmacol. 1995;280:159-66.
Menzaghi F, Whelan K, Risbrough V, Rao T, Lloyd G. Interactions between a novel cholinergic ion channel agonist, SIB-1765F and L-DOPA in the reserpine model of Parkinson’s disease in rats. J. Pharmacol Exp Ther. 1997;280:393-401.
Foley P, Gerlach M, Youdim M, Riederer P. MAO-B inhibitors: Multiple roles in the therapy of neurodegenerative disorders? Parkinsonism Relat Disord. 2000;6;25-47. https://doi.org/10.1016/S1353-8020(99)00043-7
Fernández H, Chen J. Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy. 2007;27:174S-85S. https://doi.org/10.1592/phco.27.12part2.174S
Fisher A, Biggs C, Eradiri O, Starr M. Dual effects of L-3,4-dihydroxyphenylalanine on aromatic L-amino acid decarboxylase, dopamine release and motor stimulation in the reserpine-treated rat: Evidence that behavior is dopamine independent. Neuroscience. 2000;95:97-111. https://doi.org/10.1016/S0306-4522(99)00406-6
Haleem DJ, Inam QU, Haleem MA. Effects of clinically relevant doses of methyphenidate on spatial memory, behavioral sensitization and open field habituation: A time related study. Behav Brain Res. 2015;281:208-14. https://doi.org/10.1016/j.bbr.2014.12.031
Deacon RM, Koros E, Bornemann KD, Rawlins JN. Aged Tg2576 mice are impaired on social memory and open field habituation tests. Behav Brain Res. 2009;197:466-8. https://doi.org/10.1016/j.bbr.2008.09.042
Wang X, Han C, Xu Y, Wu K, Chen S, Hu M, et al. Synthesis and evaluation of phenylxanthine derivatives as potential dual A2AR antagonists/MAO-B inhibitors for Parkinson’s disease. Molecules. 2017;22:1-13. https://doi.org/10.3390/molecules22061010
Duty S, Jenner P. Animal models of Parkinson´s disease: A source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357-91. https://doi.org/10.1111/j.1476-5381.2011.01426.x
Bishnoi M, Chopra K, Kulkarni S. Involvement of adenosinergic receptor system in an animal model of tardive dyskinesia and associated behavioural, biochemical and neurochemical changes. Eur J Pharmacol. 2006;552:55-66. https://doi.org/10.1016/j.ejphar.2006.09.010
Bishnoi M, Chopra K, Kulkarni S. Possible anti-oxidant and neuroprotective mechanisms of zolpidem in attenuating typical anti-psychotic-induced orofacial dyskinesia -A biochemical and neurochemical study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1130-8. https://doi.org/10.1016/j.pnpbp.2007.04.007
Martins M, Petronilho F, Gomes K, Dai-Pizzol F, Streck E, Quevedo J. Antipsychotic induced oxidative stress in rat brain. Neurotox Res. 2008;13:63-9. https://doi.org/10.1007/BF03033368
Naidu P, Singh A, Kulkarni S. Quercetin, a bioflavonoid attenuated haloperidol induced orofacial dyskinesia. Neuropharmacology. 2003;44:1100-6. https://doi.org/10.1016/S0028-3908(03)00101-1
Singh A, Naidu P, Kulkarni S. Possible antioxidant and neuroprotective mechanisms of FK506 in attenuating haloperidol-induced orofacial dyskinesia. Eur J Pharmacol. 2003;477:87-94. https://doi.org/10.1016/S0014-2999(03)02124-1
Pavshintsev VV, Podshivalova LS, Frolova OY, Belopolskaya OA, Averina OA, Kushnir EA, et al. Effects of mitochondrial antioxidant SkQ1 on biochemical and behavioural parameters in a Parkinsonism model in mice. Biochemistry (Mosc). 2003;82:1513-20. https://doi.org/10.1134/S0006297917120100
Molina-Jiménez M F, Sánchez-Reus M I, Benedi J. Effect of fraxetin and myricetin on rotenone-induced cytotoxicity in SH-SY5Y cells: Comparison with N-acetylcysteine. Eur J Pharmacol. 2003;472:81-7. https://doi.org/10.1016/S0014-2999(03)01902-2
Kong LD, Tan RX, Woo AY, Cheng CH. Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: Implications for the treatment of affective disorders. Pharmacol Toxicol. 2001;88:75-80.
Algunos artículos similares:
- Jorge E. Machado-Alba, Cristhian David Morales-Plaza, Patrones de prescripción de antipsicóticos en pacientes afiliados al Sistema General de Seguridad Social en Salud de Colombia , Biomédica: Vol. 33 Núm. 3 (2013)
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |