Microscopic and molecular evaluation of Strongyloides venezuelensis in an experimental life cycle using Wistar rats
Abstract
Introduction: Strongyloides venezuelensis is a nematode whose natural host is rats. It is used as a model for the investigation of human strongyloidiasis caused by S. stercoralis. The latter is a neglected tropical disease in Ecuador where there are no specific plans to mitigate this parasitic illness.
Objective: To evaluate the stages of S. venezuelensis in an experimental life cycle using Wistar rats.
Materials and methods: Male Wistar rats were used to replicate the natural biological cycle of S. venezuelensis and describe its morphometric characteristics, as well as its parasitic development. Furthermore, the production of eggs per gram of feces was quantified using two diagnostic techniques and assessment of parasite load: Kato-Katz and qPCR.
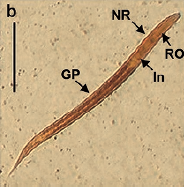
Results: Viable larval stages (L1, L2, L3) could be obtained up to 96 hours through fecal culture. Parthenogenetic females were established in the duodenum on the fifth day postinfection. Fertile eggs were observed in the intestinal tissue and fresh feces where the production peak occurred on the 8th. day post-infection. Unlike Kato-Katz, qPCR detected parasitic DNA on days not typically reported.
Conclusions: The larval migration of S. venezuelensis within the murine host in an experimental environment was equivalent to that described in its natural biological cycle. The Kato-Katz quantitative technique showed to be quick and low-cost, but the qPCR had greater diagnostic precision. This experimental life cycle can be used as a tool for the study of strongyloidiasis or other similar nematodiasis.
Downloads
References
Buonfrate D, Mena MA, Angheben A, Requema-Méndez A, Muñoz J, Gobbi F, et al. Prevalence of strongyloidiasis in Latin America: A systematic review of the literature.
Epidemiol Infect. 2015;143:452-60. https://doi.org/10.1017/S0950268814001563
Viney ME, Lok JB. The biology of Strongyloides spp. Wormbook: The online review of C. elegans biology. 2015:1-17. Access date: 11-18-2019. Available from: https://doi.org/10.1895/wormbook.1.141.2
Jacobsen KH, Ribeiro PS, Quist BK, Rydbeck BV. Prevalence of intestinal parasites in young Quichua children in the highlands of rural Ecuador. J Health Popul Nutr. 2007;25:399-405.
Reyes-Chacón JA. Evaluación del RT-PCR en el diagnóstico de 6 parásitos intestinales en un área con parasitismo de baja intensidad en el trópico, Ecuador. Quito: Universidad San Francisco de Quito; 2012. Available from: https://repositorio.usfq.edu.ec/handle/23000/2043
Júnior AF, Gonçalves-Pires MR, Silva DA, Gonçalves ALR, Costa-Cruz JM. Parasitological and serological diagnosis of Strongyloides stercoralis in domesticated dogs from southeastern Brazil. Vet Parasitol. 2006;136:137-45. https://doi.org/10.1016/j.vetpar.2005.10.022
Mati VLT, Raso P, de Melo AL. Strongyloides stercoralis infection in marmosets: Replication of complicated and uncomplicated human disease and parasite biology. Parasit Vectors. 2014;7:579. https://doi.org/10.1186/s13071-014-0579-2
Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:263-73. https://doi.org/10.1017/S0031182016000834
Ruano AL. Óxido nítrico como modulador de la estrongiloidosis. Salamanca: Universidad de Salamanca; 2008. Available from: https://dialnet.unirioja.es/servlet/tesis?codigo=102628
Viney M, Kikuchi T. Strongyloides ratti and S. venezuelensis-rodent models of Strongyloides infection. Parasitology. 2017;144:285-94. https://doi.org/10.1017/S0031182016000020
Chaves LA, Gonçalves ALR, Paula FM, Silva NM, Silva CV, Costa-Cruz JM, et al. Comparison of parasitological, immunological and molecular methods for evaluation of faecal samples of immunosuppressed rats experimentally infected with Strongyloides venezuelensis. Parasitology. 2015;142:1715-21. https://doi.org/10.1017/S0031182015001298
Marques PD, Malta FM, Meisel DMCL, Corral MA, Pinho JR, Costa-Cruz JM, et al. Diagnosis of the strongyloid nematode Strongyloides venezuelensis in experimentally infected rats. J Helminthol. 2016;90:422-7. https://doi.org/10.1017/S0022149X15000528
Viney M. Strongyloides. Parasitology. 2017;144:259-62. https://doi.org/10.1017/S0031182016001773
Bosqui LR, Marques PD, de Melo GB, Gonçalves-Pires MR, Malta FM, Pavanelli WR, et al. Molecular and immune diagnosis: Further testing for human strongyloidiasis. Mol Diagn Ther. 2018;22:485-91. https://doi.org/10.1007/s40291-018-0340-1
Organización Mundial de la Salud. Medios auxiliares para el diagnóstico de las parasitosis intestinales. Ginebra: OMS; 1994. Available from: https://www.paho.org/es/documentos/medios-auxiliares-para-diagnostico-parasitosis-intestinales
Girard de Kaminsky R. Métodos para laboratorios de atención primaria de salud. Segunda edición. Tegucigalpa: Universidad Nacional Autónoma de Honduras y Hospital-Escuela; 2003. Available from: http://www.bvs.hn/Honduras/pdf/Manual%20Parasitologia%202007.pdf
Rugai E, Mattos T, Brisola AP. A new technic for the isolation of nematode larvae from faeces; modification of Baermann’s method. Rev Inst Adolfo Lutz. 1954;14:5-8.
Verweij JJ, Canales M, Polman K, Ziem J, Brienen EAT, Polderman AM, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103:342-46. https://doi.org/10.1016/j.trstmh.2008.12.001
Beaver PCh, Jung RC, Cupp EW. Rhabditida: Strongyloides y formas relacionadas. Parasitología clínica de Craig Faust. Tercera edición. México DF: Masson Doyma S. A.; 2003.
Mehlhorn H. Encyclopedia of parasitology. Third edition. New York: Springer. 2008.
Marra NM, Chiuso-Minicucci F, Machado GC, Zorzella-Pezavento SF, França TG, Ishikawa LL, et al. Migratory route of Strongyloides venezuelensis in Lewis rats: Comparison of histological analyses and PCR. Exp Parasitol. 2011;127:334-9. https://doi.org/10.1016/j.exppara.2010.08.006
Gonçalves ALR, Silva CV, Ueta MT, Costa-Cruz JM. Antigen, antibody and immune complex detection in serum samples from rats experimentally infected with Strongyloides venezuelensis. Exp Parasitol. 2012;130:205-8. https://doi.org/10.1016/j.exppara.2012.01.007
Paula FM, Sitta RB, Malta FM, Gottardi M, Corral MA, Gryschek RCB, et al. Parasitological and molecular diagnosis in experimental Strongyloides venezuelensis infection. Rev Inst Med Trop São Paulo. 2013;55:141-3. https://doi.org/10.1590/S0036-46652013000200015
Vieira FM, Lima SS, Bessa EC. Morfologia e biometria de ovos e larvas de Strongyloides sp. grassi, 1879 (Rhabditoidea: Strongyloididae) parasito gastrointestinal de Hydrochaeris hydrochaeris (Linnaeus, 1766) (Rodentia: Hydrochaeridae), no Município de Juiz de Fora, Minas Gerais. Rev Bras Parasitol Vet. 2006;15:7-12.
El-Malky M, Maruyama H, Hirabayashi Y, Shimada S, Yoshida A, Amano T, et al. Intraepithelial infiltration of eosinophils and their contribution to the elimination of adult intestinal nematode, Strongyloides venezuelensis in mice. Parasitol Int. 2003;52:71-9. https://doi.org/10.1016/s1383-5769(02)00086-7
El-Malky MA, Maruyama H, Al-Harthi SA, El-Beshbishi SN, Ohta N. The role of B-cells in immunity against adult Strongyloides venezuelensis. Parasit Vectors. 2013;6:148. https://doi.org/10.1186/1756-3305-6-148
Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, et al. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast celldependent type 2 innate immunity. J Exp Med. 2005;202:607-16. https://doi.org/10.1084/jem.20042202
Takamure A. Migration route of Strongyloides venezuelensis in rodents. Int J Parasitol. 1995;25:907-11. https://doi.org/10.1016/0020-7519(95)00014-s
Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425-56. https://doi.org/10.1146/annurev.immunol.21.120601.141142
Gabriel JA, Rueda MM, Canales M, Sánchez A. Utilidad del método Kato-Katz para diagnóstico de uncinarias: experiencia en una zona rural de Honduras, 2011. Rev Med Hondur. 2012;80:96-101.
Easton AV, Oliveira RG, Walker M, O’Connell EM, Njenga SM, Mwandawiro ChS, et al. Sources of variability in the measurement of Ascaris lumbricoides infection intensity by Kato-Katz and qPCR. Parasit Vectors. 2017;10:256. https://doi.org/10.1186/s13071-017-2164-y
Gonçalves ALR, Silva CV, Carvalho EFG, Ueta MT, Costa-Cruz JM. Transmammary transmission of strongyloidiasis in immunosuppressed rats. Neotrop Helminthol. 2013;7:195-200. https://doi.org/10.2307/3285443
Some similar items:
- Marlene Reyes, Víctor Manuel Angulo, Life cycle of Triatoma dimidiata Latreille, 1811 (Hemiptera, Reduviidae) under laboratory conditions: production of nymphs for biological tests , Biomedica: Vol. 29 No. 1 (2009)
- Andrea Arévalo, Julio César Carranza, Felipe Guhl, Jairo Alfonso Clavijo, Gustavo Adolfo Vallejo, Comparison of the life cycles of Rhodnius colombiensis Moreno, Jurberg & Galvão, 1999 and R. prolixus Stal, 1872 (Hemiptera, Reduviidae, Triatominae) under laboratory conditions , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Sunny Sánchez, Dolores Zambrano, Maylen García, César Bedoya, Carlos Fernández, María Teresa Illnait-Zaragozí, Molecular characterization of Cryptococcus neoformans isolates from HIV patients, Guayaquil, Ecuador , Biomedica: Vol. 37 No. 3 (2017)
- Patricia Jiménez, Karina Calvopiña, Diana Herrera, Carlos Rojas, Laura Pérez-Lago, Marcelo Grijalva, Remedios Guna, Darío García-de Viedma, Identification of the Mycobacterium tuberculosis Beijing lineage in Ecuador , Biomedica: Vol. 37 No. 2 (2017)
- Rodrigo Adán Medina-Pinto, Roger Iván Rodríguez-Vivas, Manuel Emilio Bolio-González, Zoonotic intestinal nematodes in dogs from public parks in Yucatán, México , Biomedica: Vol. 38 No. 1 (2018)
- Lizbeth Díaz, Karen Covarrubias, Ángel Licón, Mixtli Astorga, Yaneth Moreno, José Alejandro Martínez, Biological parameters of Meccus phyllosomus phyllosomus (Burmeister), 1835, Triatoma recurva (Stål), 1868 (Hemiptera, Reduviidae) and their laboratory hybrids , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Nelly Solfania Heredia, Ann Sabrina Ávila, Luz Elena Velásquez, In vitro culture of L3 larvae of nematodes obtained from the African giant snail Lissachatina fulica (Mollusca: Gastropoda) in Santa Fe de Antioquia , Biomedica: Vol. 38 No. Sup. 2 (2018): Suplemento 2, Medicina tropical
- Jazzmín Arrivillaga-Henríquez, Sandra Enríquez, Vanessa Romero, Gustavo Echeverría, Jorge Pérez-Barrera, Ana Poveda, Juan-Carlos Navarro, Alon Warburg, Washington Benítez, Eco-epidemiological aspects, natural detection and molecular identification of Leishmania spp. in Lutzomyia reburra, Lutzomyia barrettoi majuscula and Lutzomyia trapidoi , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Giovani Marcelo Ramón, Rodolfo Pérez, Pablo Jarrín, Francisco Campos-Rivadeneira and Roberto Levi- Castillo: Their lives and contributions to the study of mosquitoes (Diptera: Culicidae) in Ecuador , Biomedica: Vol. 39 No. Sp. 1 (2019): Suplemento 1, Microbiología médica, mayo
- John A. Patiño, Mario J. Olivera, Gastro-allergic anisakiasis: The first case reported in Colombia and a literature review , Biomedica: Vol. 39 No. 2 (2019)
Funding data
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |