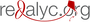La quercetina disminuye la inflamación en la región CA1 del hipocampo en un modelo de ratón triple transgénico para la enfermedad de Alzheimer.
Resumen
Introducción. La enfermedad de Alzheimer es la forma más común de demencia; se caracteriza por la presencia de marcadores histopatológicos, como las placas seniles y los ovillos neurofibrilares, así como por una activación concomitante de células microgliales y astrocitos que liberan mediadores proinflamatorios, como IL-1β, iNOS y COX-2, lo cual conduce a la disfunción y la muerte neuronal.
Objetivo. Evaluar el efecto de la quercetina sobre la reacción inflamatoria en el área CA1 del hipocampo en un modelo de ratones 3xTg-AD.
Materiales y métodos. Los animales se inyectaron intraperitonealmente con quercetina cada 48 horas durante tres meses, y se hicieron estudios histológicos y bioquímicos.
Resultados. Se encontró que en los animales 3xTg-AD tratados con quercetina, la microglía reactiva y la intensidad de fluorescencia de los agregados Aβ disminuyeron significativamente, y que hubo una menor reacción de GFAP, iNOS y COX-2, así como una clara tendencia a la reducción de la IL-1 β en lisados de hipocampo.
Conclusión. Los resultados del estudio sugieren un efecto antiinflamatorio de la quercetina en la región CA1 del hipocampo en un modelo en ratón triple trasgénico para la enfermedad de Alzheimer.
Descargas
Referencias bibliográficas
Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329-44. https://doi.org/10.1056/NEJMra0909142
Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:1-9 https://doi.org/10.3389/fncel.2014.00112
Baron R, Babcock AA, Nemirovsky A, Finsen B, Monsonego A. Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer’s disease. Aging Cell. 2014;13:584-95. https://.doi.org/10.1111/acel.12210
Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354-60.
https://doi.org/10.1523/JNEUROSCI.0616-08.2008
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2008;16:378-85. https://doi.org/10.1038/cdd.2008.172
Carrero I, Gonzalo MR, Martín B, Sanz-Anquela JM, Arévalo-Serrano J, Gonzalo-Ruiz A. Oligomers of beta-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1beta, tumour necrosis factor-alpha, and a nuclear factor kappa-B mechanism in the rat brain. Exp Neurol. 2012;236:215-27. https://doi.org/10.1016/j.expneurol.2012.05.004
Rubio-Pérez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci World J. 2012;2012:1-15. https://doi.org/10.1100/2012/756357
Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605-11.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. https://doi.org/10.1152/physrev.00029.2006
Schopfer F. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem Sci. 2003;28:646-54. https://doi.org/10.1016/j.tibs.2003.10.006
Klegeris A, Walker DG, Mcgeer PL. Activation of macrophages by Alzheimer β amyloid peptide. Biochem Biophys Res Commun. 1994;199:984-91. https://doi.org/10.1006/bbrc.1994.1326
Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117:919-47. https://doi.org/10.1007/s00702-010-0438-z
Zhang D, Hu X, Qian L, Wilson B, Lee C, Flood P, et al. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol Appl Pharmacol. 2009;238:64-70. https://doi.org/10.1016/j.taap.2009.04.015
Nagano T, Kimura SH, Takemura M. Prostaglandin E2 reduces amyloid β-induced phagocytosis in cultured rat microglia. Brain Res. 2010;1323:11-7. https://doi.org/10.1016/j.brainres.2010.01.086
Kanter M, Unsal C, Aktas C, Erboga M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol Ind Health. 2013;32:541-50. https://doi.org/10.1177/0748233713504810
Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ(1-42): Relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269-75. https://doi.org/10.1016/j.jnutbio.2008.03.002
Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodríguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134-45. https://doi.org/10.1016/j.neuropharm.2015.01.027
Aronica E, Dickson D, Kress Y, Morrison J, Zukin R. Nonplaque dystrophic dendrites in Alzheimer hippocampus: A new pathological structure revealed by glutamate receptor immunocytochemistry. Neuroscience. 1998;82:979-91. https://doi.org/10.1016/S0306-4522(97)00260-1
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles. Neuron. 2003;39:409-21.
https://doi.org/10.1016/S0896-6273(03)00434-3
Gutiérrez-Vargas J, Castro-Álvarez JF, Velásquez-Carvajal D, Montañez-Velásquez MN, Céspedes-Rubio Á, Cardona-Gómez GP. Rac1 activity changes are associated with neuronal pathology and spatial memory long-term recovery after global cerebral ischemia. Neurochem Int. 2010;57:762-73. https://doi.org/10.1016/j.neuint.2010.08.014
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918-34. https://doi.org/10.1016/j.cell.2010.02.016
von Bernhardi R, Ramírez G, Toro R, Eugenín J. Pro-inflammatory conditions promote neuronal damage mediated by Amyloid Precursor Protein and decrease its phagocytosis and degradation by microglial cells in culture. Neurobiol Dis. 2007;26:153-64. https://doi.org/10.1016/j.nbd.2006.12.006
Wang P, Guan P-P, Wang T, Yu X, Guo J-J, Wang Z-Y. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1β and Aβ between glial and neuron cells. Aging Cell. 2014;13:605-15. https://doi.org/10.1111/acel.12209
Quan Y, Jiang J, Dingledine R. EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem. 2013;288:9293-302. https://doi.org/10.1074/jbc.M113.455816
Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, et al. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Invest. 2015;125:350-64. https://doi.org/10.1172/JCI77487
Dá Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev. 2016;68:547-62. https://doi.org/10.1016/j.neubiorev.2016.06.014
Heppner FL, Ransohoff RM, Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358-72. https://doi.org/10.1038/nrn3880
Steele ML, Robinson SR. Reactive astrocytes give neurons less support: Implications for Alzheimer’s disease. Neurobiol Aging. 2012;33:423. https://doi.org/10.1016/j.neurobiolaging.2010.09.018
Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, et al. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol Aging. 2014;35:2746-60. https://doi.org/10.1016/j.neurobiolaging.2014.06.004
Kang C-H, Choi YH, Moon S-K, Kim W-J, Kim G-Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol. 2013;17:808-13. https://doi.org/10.1016/j.intimp.2013.09.009
Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, et al. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res Bull. 2007;73:55-63. https://doi.org/10.1016/j.brainresbull.2007.01.016
Lu J, Wu D, Zheng Y, Hu B, Zhang Z, Shan Q, et al. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J Pathol. 2010;222:199-212. https://doi.org/10.1002/path.2754
Sung M-S, Lee E-G, Jeon H-S, Chae H-J, Park SJ, Lee YC, et al. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation. 2012;35:1585-94. https://doi.org/10.1007/s10753-012-9473-2
Lavoie S, Chen Y, Dalton TP, Gysin R, Cuénod M, Steullet P, et al. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: Importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009;108:1410-22. https://doi.org/10.1111/j.1471-4159.2009.05908.x
Chen JC, Ho FM, Pei-Dawn LC, Chen C-P, Jeng K-CG, Hsu HB, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9-20. https://doi.org/10.1016/j.ejphar.2005.08.005
Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, et al. Functional impairment of microglia coincides with beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. https://doi.org/10.1371/journal.pone.0060921
Zhang X, Hu J, Zhong L, Wang N, Yang L, Liu C-C, et al. Quercetin stabilizes apolipoprotein E and reduces brain Aβ levels in amyloid model mice. Neuropharmacology. 2016;108:179-92. https://doi.org/10.1016/j.neuropharm.2016.04.032
Kong Y, Li K, Fu T, Wan C, Zhang D, Song H, et al. Quercetin ameliorates Aβ toxicity in dosophila AD model by modulating cell cycle-related protein expression. Oncotarget. 2016;7:67716-31. https://doi.org/10.18632/oncotarget.11963
Algunos artículos similares:
- Lina María De los Reyes, Ángel Enrique Céspedes, La combinación de atorvastatina y meloxicam inhibe la neuroinflamación y atenúa el daño celular en la isquemia cerebral experimental por embolia arterial , Biomédica: Vol. 34 Núm. 3 (2014)
- Jeimmy Cerón, Julieta Troncoso, Alteraciones de las células de la microglía del sistema nervioso central provocadas por lesiones del nervio facial , Biomédica: Vol. 36 Núm. 4 (2016)
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |